


Limestone
What you need to know
Reflections and Exam tips
Limestone and its uses
Limestone is mainly composed of the compound calcium carbonate (CaCO3). Limestone is a sedimentary rock which was formed from the shells of sea creatures that lived millions of years ago.
Limestone is quarried and can be used to produce cement, glass and concrete.
Cement
Limestone is heated with clay to make cement in a rotary kiln. The rotary kiln is heated to about 900°C and the calcium carbonate undergoes thermal decomposition to produce calcium oxide.
calcium carbonate → calcium oxide + carbon dioxide
CaCO3 → CaO + CO2
The energy costs are high and this produces carbon dioxide (CO2). This contributes to global warming and climate change.
Mortar
Cement is mixed with sand to make mortar.
Concrete
Cement is mixed with sand and aggregate to make concrete which can resist crushing or squashing forces. The concrete can be strengthened by adding steel rods to form reinforced concrete.
The Lime Cycle
Calcium carbonate
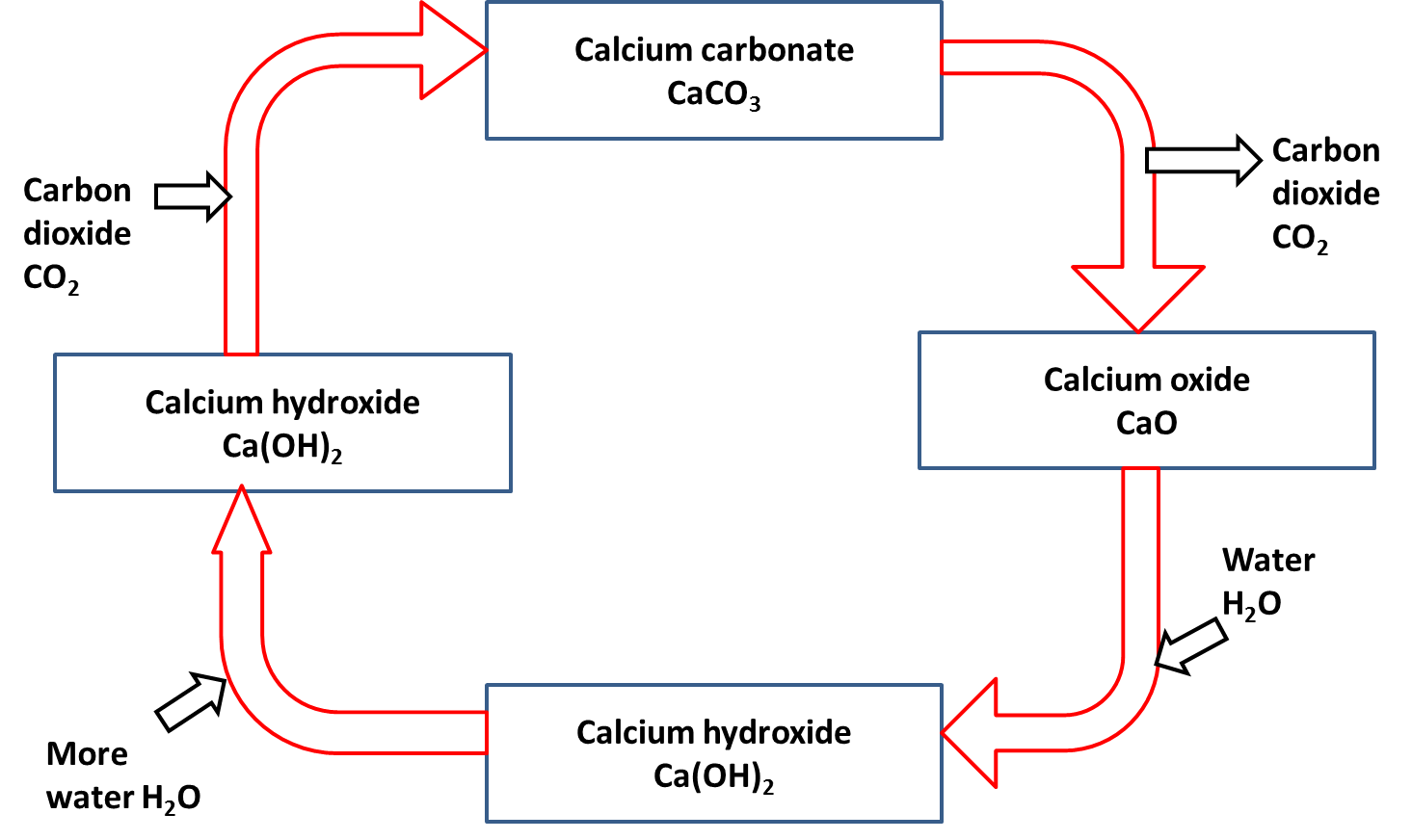
Calcium carbonate can be decomposed by heating (thermal decomposition) to make calcium oxide and carbon dioxide.
calcium carbonate → calcium oxide + carbon dioxide
CaCO3 → CaO + CO2
The carbonates of magnesium, copper, and zinc decompose on heating in a similar way. However, the more reactive the metal carbonate is the more heat energy is required.
Calcium oxide
Calcium oxide (slaked lime) reacts with water to produce calcium hydroxide (limewater).
calcium oxide + water → calcium hydroxide
CaO(s) + H2O(l) → Ca(OH)2(s)
Calcium hydroxide is an alkali that can be used in the neutralisation of acids.
Calcium hydroxide
A solution of calcium hydroxide (limewater) reacts with carbon dioxide to produce calcium carbonate.
calcium hydroxide solution + carbon dioxide → calcium carbonate + water
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
Limewater (calcium hydroxide) is used as a test for carbon dioxide. Carbon dioxide turns limewater cloudy/milky.
Reaction of carbonates
Carbonates react with acids to produce a salt, carbon dioxide and water. For example:
Calcium carbonate + hydrochloric acid → calcium chloride + carbon dioxide + water.
Limestone is damaged by acid rain.
Limestone issues
Limestone Quarrying
In order to get the limestone, explosives are used to blast it from quarries. Transported using large lorries to be processed.
| Advantages | Disadvantages |
|---|---|
Local jobs (directly and indirectly) which improves local economy Limestone is supplied to industry (iron extraction, added to bread, cosmetics, paper) Improve infrastructure (road links/by-pass) After use the quarry can be re-beautified and used for leisure activities (recreational lakes) Revenue/jobs maintained after quarry closes |
Many sites are located in National Parks. Areas of habitat are destroyed Some sites have unique/rare wildlife Blasting scares off wildlife Increased traffic noise Increased air pollution from lorries and explosions Increased risk of road accidents Dust pollution to nearby properties as well as affecting yield |
Describe limestone and its uses
Describe thermal decomposition of limestone
Describe what happens when calcium oxide reacts with water
Draw a diagram to represent the limestone cycle
Describe what happens at a quarry
Identify problems associated with quarrying
Consider the views of different people affected by quarrying